Teva and MedinCell looking to enter LAI schizophrenia market - Pharma Technology Focus | Issue 103 | February 2021

FDA declines to approve Teva-MedinCell's risperidone injection for treating schizophrenia | Seeking Alpha
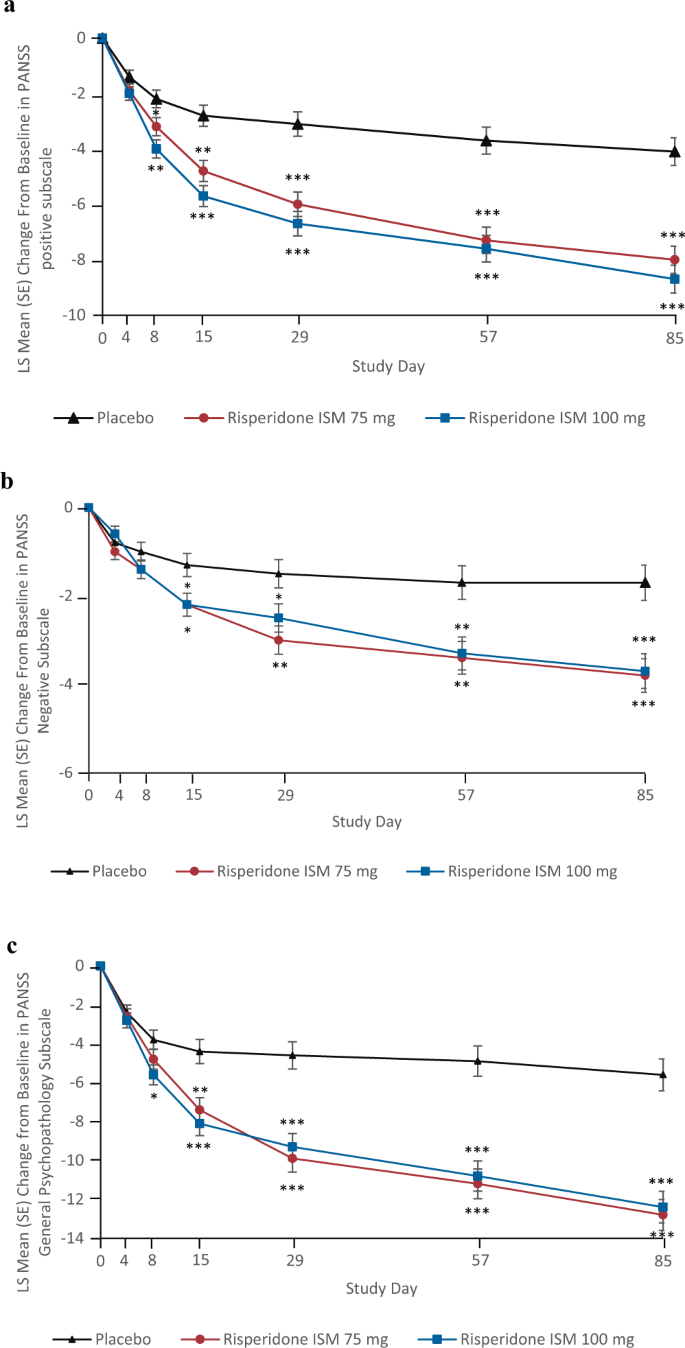
Efficacy and safety of once-monthly Risperidone ISM® in schizophrenic patients with an acute exacerbation | Schizophrenia

Teva and MedinCell Announce Positive Results for Registration Trial of Investigational Extended-Release Subcutaneous Injectable Risperidone for Patients with Schizophrenia | Business Wire

Teva and MedinCell Announce FDA Acceptance of New Drug Application for TV-46000/mdc-IRM as a Treatment for Patients with Schizophrenia | Business Wire