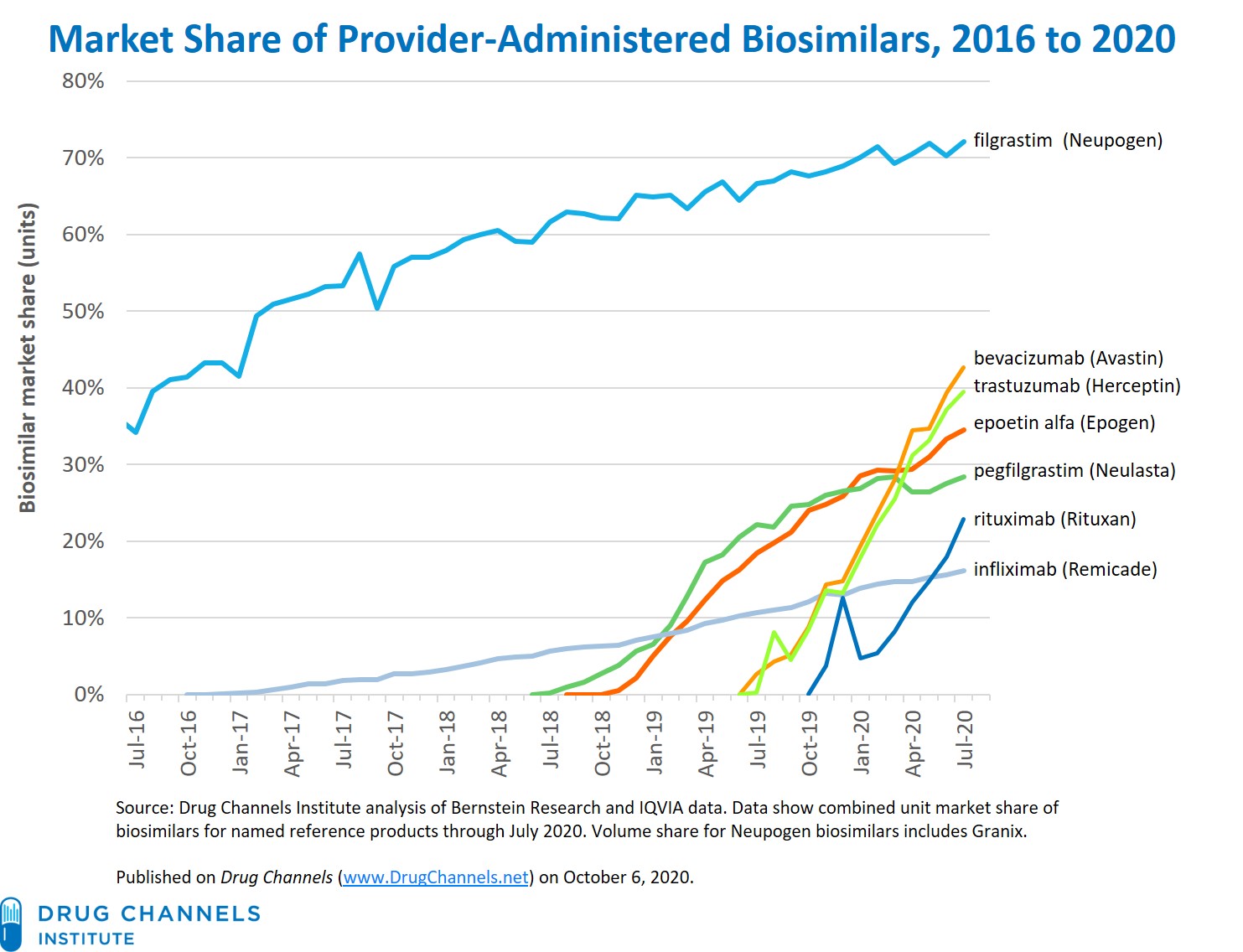
Teva and Celltrion Announce the Availability of TRUXIMA® (rituximab-abbs) Injection, the First Biosimilar to Rituxan® (rituximab) in the United States | Business Wire

Teva and Celltrion Launch Truxima (biosimilar- rituximab) to Treat Wegener's Granulomatosis and Microscopic Polyangiitis in the US

Teva Signs an Exclusive Commercialization Agreement with Bioeq for FYB201 ( biosimilar- ranibizumab)